Slides Structural Heart Disease Congenital Heart Disease
| Percutaneous Closure of Atrial Septal Defect |
||||||
|
• What type of atrial septal defect is suitable for the device closure? • What age can be indicated? • Long-term treatment and follow-up |
||||||
| What type of atrial septal defect is suitable for the device closure? | ||||||
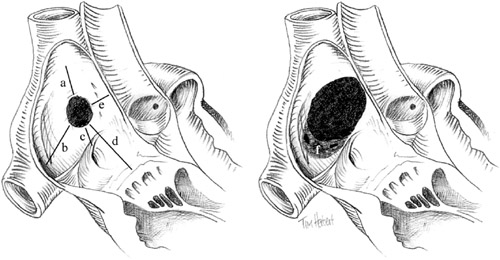
For obvious anatomical reasons, primum ASDs and sinus venosus type ASDs with anomalous pulmonary venous drainage are not suitable for percutaneous closure. Fortunately, most secundum ASDs are suitable for closure with an arial septal occluder (ASO). The ideal ASD for percutaneous closure is less than 20 mm in diameter and has firm and adequately sized margins to the mitral valve, the base of the aorta, and the orifices of the venae cavae and the coronary sinus to accommodate the atrial flanges (Figure 1, left). Such an ASD would be suitable for closure by any currently available device. Unfortunately, many ASDs, particularly in adults, are larger, have deficient margins to the aorta and coronary sinus and flimsy infero-posterior margins (Figure 1, right). Nevertheless, they may still be suitable for closure with an Amplatzer device. In practice, we attempt percutaneous closure of all secundum ASDs, provided they are less than 40 mm in diameter and have a margin of greater than 5 mm from the mitral valve, the orifices of the venae cavae, and the right upper pulmonary vein. A lesser margin (2-3 mm) to the coronary sinus is acceptable as the orifice of this structure is sufficiently large to accommodate some overlap of the device without interference to flow. Figure 1. Atrial septal defect with optimal (left) and suboptimal (right) characteristics for percutaneous closure. Rims to the superior vena cava (a), inferior vena cava (b), coronary sinus (c), tricuspid valve (d), and aorta (e). A fenestrated infero-posterior rim (f).
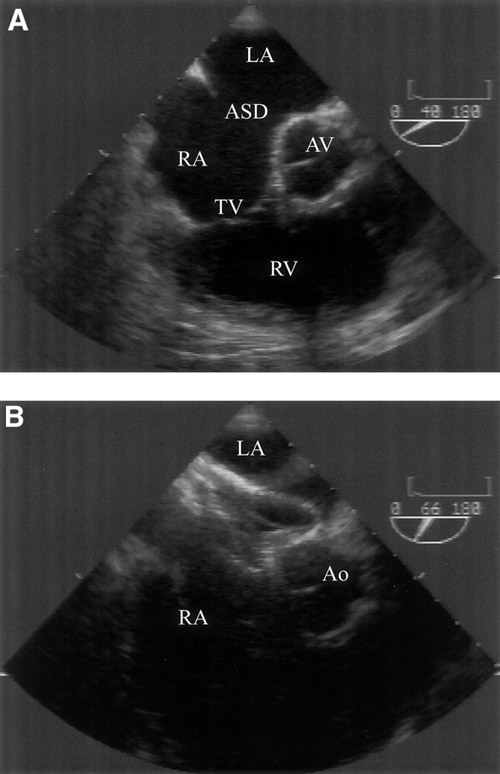
A good example of the versatility of the ASO relates to the antero-superior (aortic) rim. In the absence of an aortic rim, the ASO, if correctly sized, is sufficiently flexible to mould itself around the aortic wall with minimal risk of perforation (Figure 3), whereas more rigid devices or devices with sharp edges would carry the risk of subacute perforation. In selected cases, more than one ASD or fenestrated ASDs in association with aneurysmal septal tissue can be closed with an ASO or PFO occluder or combination of two devices. Figure 2. Atrial septal defect (ASD) with deficient aortic rim prior to device deployment (A) and postdeployment with the Amplatzer septal occluder device splayed around aortic root (B). Left atrium (LA), right atrium (RA), aortic valve (AV), tricuspid valve (TV), right ventricle (RV), and aorta (Ao).
Assessment of suitability for percutaneous closure is best done by transthoracic (TTE) and transesophageal (TEE) echocardiography. Three-dimensional echocardiography obtained from TEE images, if available, may provide anatomical detail, but the accuracy of the reconstructed images are heavily dependent on the technical expertise of the echocardiographer. |
||||||
| What age can be indicated? | ||||||
| With growth, ASDs tend to increase in size approximately in proportion to increase in body size. ASDs therefore tend to be larger in adults than in children, but the margins of the defect in relation to other structures such as the pulmonary veins on the mitral valve are also greater. Although some advocate immediate closure of ASDs with evidence of right heart volume overload irrespective of age, we prefer where possible to wait until the child has reached at least the age of 5 or is greater than 20 kg in weight. |
||||||
| Long-term treatment and follow-up | ||||||
| We recommend to perform TTE at 3, 6, and 12 months
after procedure. Aspirin therapy is advised for 48 hr preprocedure and
for 6 months postprocedure until the device is fully endothelialized.
Antibiotic prophylaxis is also recommended for procedures that would warrant
it during this period. |
||||||
| References | ||||||
|
||||||


Leave a comment