News | TCTAP & AP VALVES 2020 Virtual
Imaging-guided PSP: How to Optimize Your Stent for Complex Coronary Lesions?
August 6th, Highlight Session of TCTAP & AP VALVES 2020 VIRTUAL
Percutaneous coronary intervention (PCI) for complex coronary artery disease such as left main, bifurcation, diffusely long, and severely calcified lesions has been increasingly performed because of increased longevity of the target population, enhanced pharmacotherapies, improved stents, and techniques. Nevertheless, it is still related to lesser favorable clinical outcomes than simple-lesion PCI.
 The role of intravascular imaging for optimal stenting for complex coronary lesions has been emphasized through recent clinical studies. Jung-Min Ahn, MD (Asan Medical Center, Seoul, Republic of Korea), presented his latest research of the intravascular imaging guided optimal stenting technique for complex lesions at the highlight session of TCTAP & AP VALVES 2020 VIRTUAL.
The role of intravascular imaging for optimal stenting for complex coronary lesions has been emphasized through recent clinical studies. Jung-Min Ahn, MD (Asan Medical Center, Seoul, Republic of Korea), presented his latest research of the intravascular imaging guided optimal stenting technique for complex lesions at the highlight session of TCTAP & AP VALVES 2020 VIRTUAL.
The concept of optimal stenting was rejuvenated with the lesson from bioresorbable scaffolds intervention, needing standardizing pre-dilation, stent sizing, and post-dilation (PSP). Dr. Ahn and his colleagues introduced a concept of intravascular imaging guided PSP (iPSP), which integrating intravascular imaging to the classic PSP, and evaluated its role for patients with complex coronary artery lesions.
He analyzed a registry data including 9,525 patients who underwent complex-lesion PCI at 42 hospitals in Korea between 2008 and 2017. Complex coronary lesions included left main stenosis, bifurcation, long lesions (30 mm or over) and severely calcified lesions. The iPSP technique was defined as consisting of 3 components: 1) pre-dilation, 2) intravascular imaging-guided stent sizing, 3) post-dilation using noncompliant balloons for complete stent expansion, which was confirmed by final intravascular imaging surveillance.
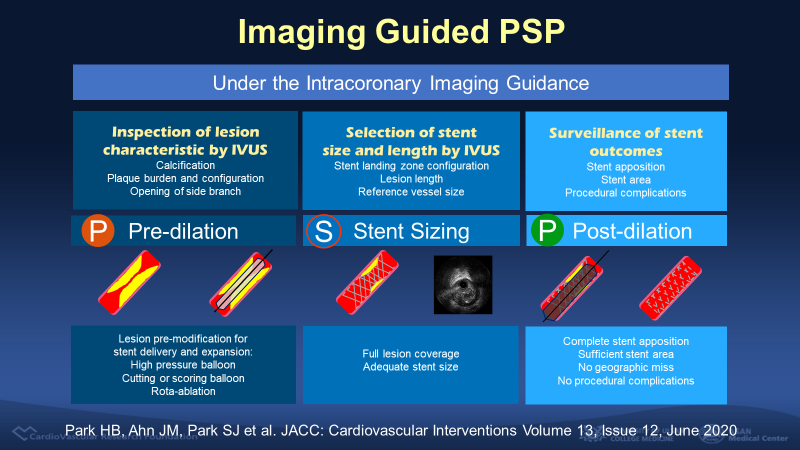
Figure 1. The definition of imaging-guided PSP (iPSP)
A complete iPSP strategy was performed in 3,374 patients (35.4%) whereas in 6,151 patients, 1 or more steps of the iPSP procedure were not performed. Patients treated according to the iPSP procedure were younger and included more men and of those who had hypertension, hyperlipidemia, and more complex and longer lesions compared with no-iPSP patients. To overcome differences between the two groups, he performed extensive statistical adjustment including propensity score-matching and inverse probability of treatment weighting (IPTW).
The patients with complete iPSP received more (1.41 vs. 1.35, p<0.001), longer (37 vs. 33 mm, p<0.001), and larger (3.3 vs. 3.2 mm, p<0.001) stents with larger final balloon size (3.6 vs. 3,4 mm, p<0.001).
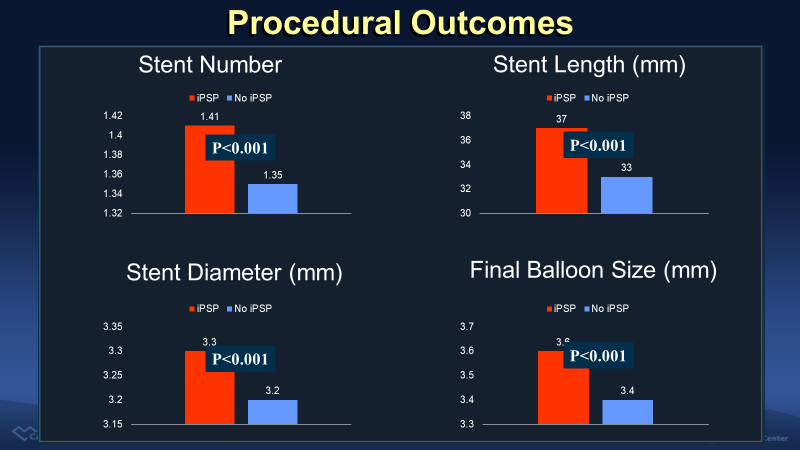
Figure 2. More, larger, and longer stents and larger final balloon was used in iPSP group.
The primary outcome, a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization at a 3-year follow up, was 5.7% in patients treated with iPSP and 8.0% with no iPSP (p<0.001). The difference was consistent after adjustment with IPTW (Hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.63-0.81, p<0.001). The rate of cardiac death (HR 0.62, 95% CI 0.51-0.75, p=0.003) and target vessel revascularization (HR 0.74, 95% CI 0.63-0.87, p<0.001) was lower in iPSP group. There was no significant difference in the target vessel myocardial infarction. The single most important prognostic factor for primary outcome was the IVUS-optimized post-dilation (HR 0.80, 95% CI 0.67-0.96, p=0.016) when the impact of each 3 step was analyzed in the complete iPSP group.
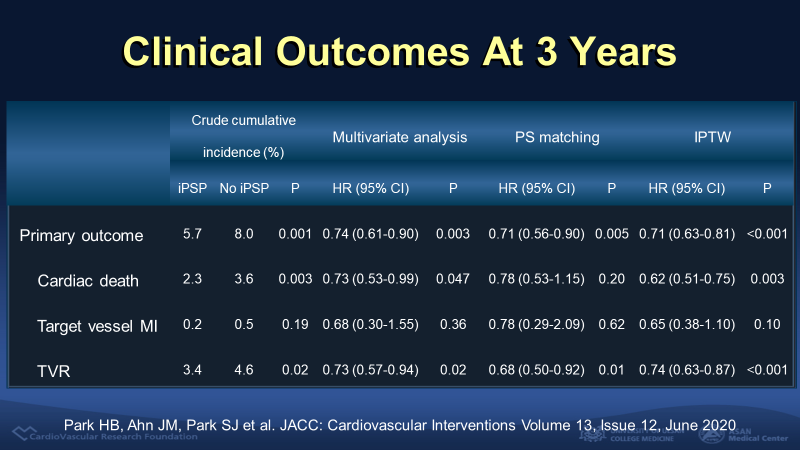
Figure 3. Clinical outcomes at 3 year according to iPSP
This study showed that iPSP strategy was associated with a significantly lower risk of adverse cardiac events n in patients with complex coronary lesions. Dr. Ahn emphasized that the clinical benefit of iPSP seems to be attributed to safe and effective post-dilation, with the larger final balloon size guided by intracoronary imaging. He said, “This study suggested that physicians should recognize the importance of iPSP strategy and more actively consider it for the treatment of complex coronary artery disease.”
A co-moderator of this highlight session, Myeong-ki Hong, MD (Severance Hospital, Seoul, South Korea) commented, “This presentation was very educational. It was the best way to persuade young interventionists without knowledge of intravascular imaging, that a larger post-dilation balloon using intravascular imaging guidance makes a larger stent area.”
“Which do you focus more between intravascular imaging guidance or PSP?” Bon-Kwon Koo (Seoul National University Hospital, Seoul, South Korea) asked to panelists.
“My personal bias is that if you do imaging, everything else follows logically, and, each steps of the procedure are optimized. Once you do imaging, the rest of it totally make sense. But to do it blinded, just with angiographic guidance, you have not really optimized it much.”, Gary S. Mintz (Cardiovascular Research Foundation, New York, USA) mentioned. The discussant made a consensus that intravascular imaging guidance can achieve more proper, larger sizing compared with non-imaging guidance. The larger stent area, about 1.5 mm2, in imaging-guided PCI group was consistently shown through many clinical studies.
“Which imaging modality is more beneficial between intravascular ultrasound (IVUS) or optical coherent tomography (OCT)?” Seung-Jung Park, MD (Asan Medical Center, Seoul, South Korea) asked.
Ziad A. Ali (Columbia University Medical Center, New York, USA) commented, “I think that OCT has two major advantages. The first advantage is that the OCT automatically measures the lumen area and vessel size. The second is that OCT is useful for the calcified lesion with measurement of calcium thickness. But I think that IVUS is a clear winner for chronic kidney disease, chronic total occluded lesion, and ostial lesion. I think that new high-definition IVUS system is very good because of achievement resolute like OCT without contrast and durability.”
Dr. Mintz finally commented, “I find the IVUS versus OCT argument or discussion to be a distraction. The main issue is imaging versus angiography.”
Edited by

Hanbit Park , MD
GangNeung Asan Hospital, Korea (Republic of)
Percutaneous coronary intervention (PCI) for complex coronary artery disease such as left main, bifurcation, diffusely long, and severely calcified lesions has been increasingly performed because of increased longevity of the target population, enhanced pharmacotherapies, improved stents, and techniques. Nevertheless, it is still related to lesser favorable clinical outcomes than simple-lesion PCI.
 The role of intravascular imaging for optimal stenting for complex coronary lesions has been emphasized through recent clinical studies. Jung-Min Ahn, MD (Asan Medical Center, Seoul, Republic of Korea), presented his latest research of the intravascular imaging guided optimal stenting technique for complex lesions at the highlight session of TCTAP & AP VALVES 2020 VIRTUAL.
The role of intravascular imaging for optimal stenting for complex coronary lesions has been emphasized through recent clinical studies. Jung-Min Ahn, MD (Asan Medical Center, Seoul, Republic of Korea), presented his latest research of the intravascular imaging guided optimal stenting technique for complex lesions at the highlight session of TCTAP & AP VALVES 2020 VIRTUAL.
The concept of optimal stenting was rejuvenated with the lesson from bioresorbable scaffolds intervention, needing standardizing pre-dilation, stent sizing, and post-dilation (PSP). Dr. Ahn and his colleagues introduced a concept of intravascular imaging guided PSP (iPSP), which integrating intravascular imaging to the classic PSP, and evaluated its role for patients with complex coronary artery lesions.
He analyzed a registry data including 9,525 patients who underwent complex-lesion PCI at 42 hospitals in Korea between 2008 and 2017. Complex coronary lesions included left main stenosis, bifurcation, long lesions (30 mm or over) and severely calcified lesions. The iPSP technique was defined as consisting of 3 components: 1) pre-dilation, 2) intravascular imaging-guided stent sizing, 3) post-dilation using noncompliant balloons for complete stent expansion, which was confirmed by final intravascular imaging surveillance.

Figure 1. The definition of imaging-guided PSP (iPSP)
A complete iPSP strategy was performed in 3,374 patients (35.4%) whereas in 6,151 patients, 1 or more steps of the iPSP procedure were not performed. Patients treated according to the iPSP procedure were younger and included more men and of those who had hypertension, hyperlipidemia, and more complex and longer lesions compared with no-iPSP patients. To overcome differences between the two groups, he performed extensive statistical adjustment including propensity score-matching and inverse probability of treatment weighting (IPTW).
The patients with complete iPSP received more (1.41 vs. 1.35, p<0.001), longer (37 vs. 33 mm, p<0.001), and larger (3.3 vs. 3.2 mm, p<0.001) stents with larger final balloon size (3.6 vs. 3,4 mm, p<0.001).

Figure 2. More, larger, and longer stents and larger final balloon was used in iPSP group.
The primary outcome, a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization at a 3-year follow up, was 5.7% in patients treated with iPSP and 8.0% with no iPSP (p<0.001). The difference was consistent after adjustment with IPTW (Hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.63-0.81, p<0.001). The rate of cardiac death (HR 0.62, 95% CI 0.51-0.75, p=0.003) and target vessel revascularization (HR 0.74, 95% CI 0.63-0.87, p<0.001) was lower in iPSP group. There was no significant difference in the target vessel myocardial infarction. The single most important prognostic factor for primary outcome was the IVUS-optimized post-dilation (HR 0.80, 95% CI 0.67-0.96, p=0.016) when the impact of each 3 step was analyzed in the complete iPSP group.

Figure 3. Clinical outcomes at 3 year according to iPSP
This study showed that iPSP strategy was associated with a significantly lower risk of adverse cardiac events n in patients with complex coronary lesions. Dr. Ahn emphasized that the clinical benefit of iPSP seems to be attributed to safe and effective post-dilation, with the larger final balloon size guided by intracoronary imaging. He said, “This study suggested that physicians should recognize the importance of iPSP strategy and more actively consider it for the treatment of complex coronary artery disease.”
A co-moderator of this highlight session, Myeong-ki Hong, MD (Severance Hospital, Seoul, South Korea) commented, “This presentation was very educational. It was the best way to persuade young interventionists without knowledge of intravascular imaging, that a larger post-dilation balloon using intravascular imaging guidance makes a larger stent area.”
“Which do you focus more between intravascular imaging guidance or PSP?” Bon-Kwon Koo (Seoul National University Hospital, Seoul, South Korea) asked to panelists.
“My personal bias is that if you do imaging, everything else follows logically, and, each steps of the procedure are optimized. Once you do imaging, the rest of it totally make sense. But to do it blinded, just with angiographic guidance, you have not really optimized it much.”, Gary S. Mintz (Cardiovascular Research Foundation, New York, USA) mentioned. The discussant made a consensus that intravascular imaging guidance can achieve more proper, larger sizing compared with non-imaging guidance. The larger stent area, about 1.5 mm2, in imaging-guided PCI group was consistently shown through many clinical studies.
“Which imaging modality is more beneficial between intravascular ultrasound (IVUS) or optical coherent tomography (OCT)?” Seung-Jung Park, MD (Asan Medical Center, Seoul, South Korea) asked.
Ziad A. Ali (Columbia University Medical Center, New York, USA) commented, “I think that OCT has two major advantages. The first advantage is that the OCT automatically measures the lumen area and vessel size. The second is that OCT is useful for the calcified lesion with measurement of calcium thickness. But I think that IVUS is a clear winner for chronic kidney disease, chronic total occluded lesion, and ostial lesion. I think that new high-definition IVUS system is very good because of achievement resolute like OCT without contrast and durability.”
Dr. Mintz finally commented, “I find the IVUS versus OCT argument or discussion to be a distraction. The main issue is imaging versus angiography.”
Edited by

Hanbit Park , MD
GangNeung Asan Hospital, Korea (Republic of)


Leave a comment