News | SEOUL VALVES 2022
Data on TAVR-related stroke 'not enough, hard to justify routine EPD use except in BAV, ViVs,'
David J. Cohen, MD says field 'just not that good' at predicting TAVR-related stroke and its effects, but EPDs help patients with bicuspid valves, ViV procedures for now
The benefit of using cerebral embolic protection devices (EPDs) to prevent stroke related to transcatheter aortic valve replacement (TAVR) remains uncertain, an expert said, but patient subgroups nevertheless stand to benefit from routine use.
 "Although EPD usage is increasing in the United States, it's difficult to justify - at the present moment - their selective use except for cases of bicuspid aortic stenosis (AS) or valve-in-valve (ViV) procedures," said David Joel Cohen, MD (Cardiovascular Research Foundation; St. Francis Hospital, New York, USA) on Aug 11 at AP VALVES & STRUCTURAL HEART 2022 held at the Grand Walkerhill Seoul in South Korea.
"Although EPD usage is increasing in the United States, it's difficult to justify - at the present moment - their selective use except for cases of bicuspid aortic stenosis (AS) or valve-in-valve (ViV) procedures," said David Joel Cohen, MD (Cardiovascular Research Foundation; St. Francis Hospital, New York, USA) on Aug 11 at AP VALVES & STRUCTURAL HEART 2022 held at the Grand Walkerhill Seoul in South Korea.

David J. Cohen, MD asks panelists on routine EPD use for preventing TAVR-related stroke during an AP VALVES & SH 2022 session held at the Grand Walkerhill Seoul in South Korea on Aug 11.
EPDs are mesh filters that clear out debris during a TAVR procedure, which are thought to prevent cerebrovascular events like major or minor stroke, transient ischemic attacks (TIA), neurocognitive decline and "silent" cerebral infarcts, which are significant but unpredictable complications of TAVR.
However, lacking concrete data on the clinical efficacy of EPDs for preventing or protecting against TAVR-related stroke and other related complications has confounded attempts to use them routinely in the clinical setting.
EPDs have been proven to capture TAVR procedure-related debris and likely reduce the volume of new brain lesions but we're just not that good at predicting stroke,
Cohen - who specializes in researching the cost-effectiveness of novel devices and procedures in interventional cardiology - noted that the problem poses a significant challenge from both clinical and economic standpoints.
Analysis on 129,000 TAVR cases from Medicare Claims in the United States from 2012 to 2017 showed that TAVR-related in-hospital stroke (4.3%) was associated with an increased risk of mortality up to 5 years and a $9,000 annual increase per patient, he said.
Several EPDs - including Sentinel (Claret Medical; Boston Scientific, Minnesota), TriGuard3 (Keystone Heart, Florida), ProtEmbo (Protembis, Germany), Emblok (Innovative Cardiovascular Solutions, US), Emboliner (Emboline, CA, USA) and Point-guard (Transverse Medical Inc, Colorado, USA) - were developed as potential solutions.
Sentinel is the only device to have gained American and European approval based on the SENTINEL IDE trial1, but approval came through for its ability to clear out debris - not for preventing stroke.
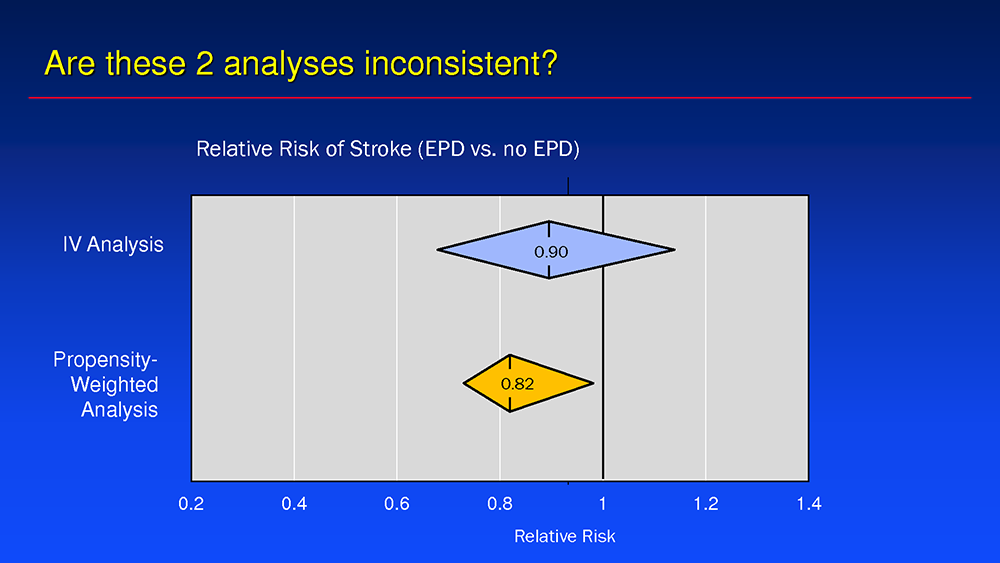
Routine use of EPDs is also contested due to major analyses, like that of the observational TVT Registry, that showed EPD’s efficacy to be "more modest than originally thought." The study2 led by Cohen and colleagues on embolic protection for TAVR examined the rate of in-hospital death or stroke in 132,248 TAVR patients treated with EPDs (n=12,409) or without (n=110,777) and employed both instrumental variable (IV) and propensity-weighted analysis.
Results of IV analysis showed a statistically non-significant trend for EPDs to reduce TAVR-related stroke (EPD 2.4% vs. no EPD 2.6%; RR 0.93, 95% CI, 0.76-1.11, p=0.47). Conversely, the propensity-weighted analysis found a small, but statistically significant stroke reduction with EPDs (1.30% vs. 1.58%; RR 0.82, 95% CI, 0.69-0.97).
Investigators concluded that embolic protection devices were "generally safe" and did not increase rates of vascular complications, major bleeding or device failure.
On the discrepancy between the two analytical approaches, Cohen explained that side-by-side comparisons showed no significant difference between them, except for a wider variance with the IV analysis.
Investigators also observed a strong trend between EPDs and stroke reduction in two subgroups:, bicuspid aortic valves (BAVs) and ViV procedures, These findings signaled the need for larger randomized controlled trials, Cohen said.
The two right answers on who needs EPDs in 2022 is everybody or nobody,
"Aside from the substantial stroke reductions for patients with bicuspid anatomy and ViVs, patient selection for stroke remains fairly challenging," he said. "Although we created the TVT Stroke Model3 to identify risk factors for stroke, it had a poor c-statistic of 0.62 - and considering a value of 0.5 would essentially be a coin flip - we concluded that the model is good for calibration but not for discrimination.
"So far, we haven’t been able to see a decline in neurocognitive function in trials, but to be fair, the trials so far have been small," he added. "We need more research on the long-term neurocognitive effects of non-disabling and clinically-silent strokes since evidence has shown emboli and asymptomatic cerebral emboli are associated with neurocognitive decline and because those would ultimately be the major targets for these devices."
The ongoing PROTECTED-TAVR (n=3,000) and BHF-PROTECT TAVI (n=7,730) studies - two large-scale randomized controlled trials (RCTs) rolled out in the US and UK, respectively - are expected to shed long-awaited answers on the efficacy of the Sentinel device and stroke prevention.
Perspectives on current, future routine EPD usage in field
When asked about routine use of EPDs in clinical practice based on existing data, Cohen quipped: "The two right answers on who needs EPDs in 2022 is everybody or nobody. There isn’t much case selection because we just cannot detect strokes that well."
"EPDs have been proven to capture TAVR procedure-related debris - and likely reduce the volume of new brain lesions. And we’re just not that good at predicting stroke," he said. "In Kansas City, I used them only for bicuspid and ViV procedures because of financial barriers but in New York - where I am now - we use them 100% except for cases of unfavorable anatomy or position.
"When the evidence is not that strong, that’s the right thing to do."
When the question was turned to session panelists, a show of hands showed only 3 out of 8 clinicians used EPDs routinely in clinical practice.
We need more research on the long-term neurocognitive effects of non-disabling and clinically-silent strokes because those would ultimately be the major targets for embolic protection devices.
If future studies were to demonstrate high prevention rates ("70-80%") for large strokes, Cohen hypothesized, then the recommendation for using EPDs would be upped to 100%, although concerns of trials like PROTECTED-TAVR being underpowered are limitations.
"Results from PROTECTED-TAVR are coming up in 5 weeks at TCT 2022 and - even though I don’t know the results yet- it’s hard to imagine a 50% reduction with these observational data - it just doesn’t seem to be there," he said.
"Even if Sentinel doesn’t reduce all strokes, a good signal seems to be emerging for large strokes, despite the problem of the studies being underpowered," he added. "If EPDs do reduces large strokes, we should be using them for everyone, because that’s compelling evidence. Regardless, I’m just glad we’re getting the evidence needed to move forward - it’s been frustrating not to have it."
Edited by

Jung-Min Ahn , MD
Asan Medical Center, Korea (Republic of)
Written by

YoonJee Marian Chu, Medical Journalist
Read Biography
Butala, Neel M., et al. "Cerebral Embolic Protection and Outcomes of Transcatheter Aortic Valve Replacement: Results from the Transcatheter Valve Therapy Registry." Circulation, vol. 143, no. 23, 2021, pp. 2229-2240., doi:10.1161/circulationaha.120.052874.
Cohen disclosed grant and drug support from Bristol Myers Squibb (MyoKardia), Edwards Lifesciences, Medtronic, Corvia Medical, I-Rhythm Technologies, Abbott Vascular, Boston Scientific, Philips Healthcare, Zoll Medical (TherOx); Consulting fees and advisory boards for Medtronic, Boston Scientific, Corvia Medical, Edwards Lifesciences, Abbott Vascular and Impulse Dynamics
The benefit of using cerebral embolic protection devices (EPDs) to prevent stroke related to transcatheter aortic valve replacement (TAVR) remains uncertain, an expert said, but patient subgroups nevertheless stand to benefit from routine use.
 "Although EPD usage is increasing in the United States, it's difficult to justify - at the present moment - their selective use except for cases of bicuspid aortic stenosis (AS) or valve-in-valve (ViV) procedures," said David Joel Cohen, MD (Cardiovascular Research Foundation; St. Francis Hospital, New York, USA) on Aug 11 at AP VALVES & STRUCTURAL HEART 2022 held at the Grand Walkerhill Seoul in South Korea.
"Although EPD usage is increasing in the United States, it's difficult to justify - at the present moment - their selective use except for cases of bicuspid aortic stenosis (AS) or valve-in-valve (ViV) procedures," said David Joel Cohen, MD (Cardiovascular Research Foundation; St. Francis Hospital, New York, USA) on Aug 11 at AP VALVES & STRUCTURAL HEART 2022 held at the Grand Walkerhill Seoul in South Korea.

EPDs are mesh filters that clear out debris during a TAVR procedure, which are thought to prevent cerebrovascular events like major or minor stroke, transient ischemic attacks (TIA), neurocognitive decline and "silent" cerebral infarcts, which are significant but unpredictable complications of TAVR.
However, lacking concrete data on the clinical efficacy of EPDs for preventing or protecting against TAVR-related stroke and other related complications has confounded attempts to use them routinely in the clinical setting.
EPDs have been proven to capture TAVR procedure-related debris and likely reduce the volume of new brain lesions but we're just not that good at predicting stroke,
Cohen - who specializes in researching the cost-effectiveness of novel devices and procedures in interventional cardiology - noted that the problem poses a significant challenge from both clinical and economic standpoints.
Analysis on 129,000 TAVR cases from Medicare Claims in the United States from 2012 to 2017 showed that TAVR-related in-hospital stroke (4.3%) was associated with an increased risk of mortality up to 5 years and a $9,000 annual increase per patient, he said.
Several EPDs - including Sentinel (Claret Medical; Boston Scientific, Minnesota), TriGuard3 (Keystone Heart, Florida), ProtEmbo (Protembis, Germany), Emblok (Innovative Cardiovascular Solutions, US), Emboliner (Emboline, CA, USA) and Point-guard (Transverse Medical Inc, Colorado, USA) - were developed as potential solutions.
Sentinel is the only device to have gained American and European approval based on the SENTINEL IDE trial1, but approval came through for its ability to clear out debris - not for preventing stroke.
Routine use of EPDs is also contested due to major analyses, like that of the observational TVT Registry, that showed EPD’s efficacy to be "more modest than originally thought." The study2 led by Cohen and colleagues on embolic protection for TAVR examined the rate of in-hospital death or stroke in 132,248 TAVR patients treated with EPDs (n=12,409) or without (n=110,777) and employed both instrumental variable (IV) and propensity-weighted analysis.
Results of IV analysis showed a statistically non-significant trend for EPDs to reduce TAVR-related stroke (EPD 2.4% vs. no EPD 2.6%; RR 0.93, 95% CI, 0.76-1.11, p=0.47). Conversely, the propensity-weighted analysis found a small, but statistically significant stroke reduction with EPDs (1.30% vs. 1.58%; RR 0.82, 95% CI, 0.69-0.97).
Investigators concluded that embolic protection devices were "generally safe" and did not increase rates of vascular complications, major bleeding or device failure.
On the discrepancy between the two analytical approaches, Cohen explained that side-by-side comparisons showed no significant difference between them, except for a wider variance with the IV analysis.

Investigators also observed a strong trend between EPDs and stroke reduction in two subgroups:, bicuspid aortic valves (BAVs) and ViV procedures, These findings signaled the need for larger randomized controlled trials, Cohen said.
The two right answers on who needs EPDs in 2022 is everybody or nobody,
"Aside from the substantial stroke reductions for patients with bicuspid anatomy and ViVs, patient selection for stroke remains fairly challenging," he said. "Although we created the TVT Stroke Model3 to identify risk factors for stroke, it had a poor c-statistic of 0.62 - and considering a value of 0.5 would essentially be a coin flip - we concluded that the model is good for calibration but not for discrimination.
"So far, we haven’t been able to see a decline in neurocognitive function in trials, but to be fair, the trials so far have been small," he added. "We need more research on the long-term neurocognitive effects of non-disabling and clinically-silent strokes since evidence has shown emboli and asymptomatic cerebral emboli are associated with neurocognitive decline and because those would ultimately be the major targets for these devices."
The ongoing PROTECTED-TAVR (n=3,000) and BHF-PROTECT TAVI (n=7,730) studies - two large-scale randomized controlled trials (RCTs) rolled out in the US and UK, respectively - are expected to shed long-awaited answers on the efficacy of the Sentinel device and stroke prevention.
Perspectives on current, future routine EPD usage in field
When asked about routine use of EPDs in clinical practice based on existing data, Cohen quipped: "The two right answers on who needs EPDs in 2022 is everybody or nobody. There isn’t much case selection because we just cannot detect strokes that well."
"EPDs have been proven to capture TAVR procedure-related debris - and likely reduce the volume of new brain lesions. And we’re just not that good at predicting stroke," he said. "In Kansas City, I used them only for bicuspid and ViV procedures because of financial barriers but in New York - where I am now - we use them 100% except for cases of unfavorable anatomy or position.
"When the evidence is not that strong, that’s the right thing to do."
When the question was turned to session panelists, a show of hands showed only 3 out of 8 clinicians used EPDs routinely in clinical practice.
We need more research on the long-term neurocognitive effects of non-disabling and clinically-silent strokes because those would ultimately be the major targets for embolic protection devices.
If future studies were to demonstrate high prevention rates ("70-80%") for large strokes, Cohen hypothesized, then the recommendation for using EPDs would be upped to 100%, although concerns of trials like PROTECTED-TAVR being underpowered are limitations.
"Results from PROTECTED-TAVR are coming up in 5 weeks at TCT 2022 and - even though I don’t know the results yet- it’s hard to imagine a 50% reduction with these observational data - it just doesn’t seem to be there," he said.
"Even if Sentinel doesn’t reduce all strokes, a good signal seems to be emerging for large strokes, despite the problem of the studies being underpowered," he added. "If EPDs do reduces large strokes, we should be using them for everyone, because that’s compelling evidence. Regardless, I’m just glad we’re getting the evidence needed to move forward - it’s been frustrating not to have it."
Edited by

Jung-Min Ahn , MD
Asan Medical Center, Korea (Republic of)
Written by



Leave a comment