|
The character strings of the whole abstract should be within 3,500 bytes including BODY, TABLE, and FIGURE, but not the title or general information of the abstract. The character counter will count only letters.
- The title of abstract should be within 255 bytes.
- An abstract must have a short, specific title (containing no abbreviations) that indicates the nature of the investigation.
- Please select one category that is closest to the subject of your abstract.
- Abstracts submitted to a category that does not match the subject matter of the abstract may receive a low score from the reviewers.
Abstracts should involve the following disciplines in the field of cardiovascular medicine and intervention.
| CORONARY |
- Antiplatelet Agents and Anticoagulants
- Bifurcation and Left Main Stenting
- Chronic Total Occlusions
- Drug-eluting Stents
- High Risk Patients: Diabetes, Heart Failure, Reanl Failure, Others
- Adjunctive Procedures: Thrombectomy, Plaque Modification, Others
- Acute Coronary Syndrome: STEMI, NSTE-ACS
- Vulnerable Plaque Diagnosis and Treatment
- Other Pharmacologic Agents
|
| ENDOVASCULAR |
- Carotid & Neurovascular Intervention
- Peripheral Vascular Intervention (Non-carotid, Non-neurovascular)
|
| STRUCTURAL HEART DISEASE |
- Congenital Heart Disease
- Left Atrial Appendage Closure
- Hypertrophic Obstructive Cardiomyopathy
- Valvular Heart Disease
- Structural Heart Disease, Other
|
| IMAGING AND PHYSIOLOGIC LESION ASSESSMENT |
- Invasive Coronary Imaging: IVUS, OCT, Spectroscopy, and Other
- Non-Invasive Cardiac Imaging: CTA, MRI, 3D-Echo, and Other
- Physiologic Lesion Assessment
|
| OTHER |
- Basic Science, Animal Models and Preclinical Studies
- Cell Therapy and Angiogenesis
- Innovative Devices and Futuristic Therapies
- Transradial Intervention
- Other (Unclassified)
|
- Please select the presentation preference( oral or e-poster) for the presentation. It may be changed at the discretion of the scientific committee.
- Only computer presentation by using LCD projector is available in the oral presentation session.
- Please select presenter'/co-author's nationality from Korean and Other.
- Please first input presenter's information and then follow co-authors' information.
- Presenter should be one who will deliver presentation of abstract when your abstract is accepted.
- You should input first and middle name in initial letter, but last name in full for presenter'/co-authors' name. (e.g.: Kyung Ae Kim -> K A Kim, John S. Valentine -> J S Valentine)
- If the abstract that you are now submitting has only one affiliation for all co-authors, you should input "0" (zero)
into number blank, No.
 . .
- If your abstract has more than two affiliations involved, then you should input Arabic numerals from "1" and in a row, following the numbers of the different affiliations. (e.g.: 1, 2, 3…).
- In case that presenter's/authors' affiliations are the same, please use the same number. If you input the same number that you once designate, you can see the linked affiliation automatically. Please note that the same number of the superscript on presenter/co-authors means the same affiliations. (e.g.: K.A. Kim1, C. Lee1, J. Gwon2 ; 1Asan Medical Center, Seoul, Korea, 2Samsung Medical Center, Seoul, Korea).
- The name of affiliations should be completed by official institution name. Provide complete city, state or province and country (e.g., Asan Medical Center, Seoul, Korea).
- You can check immediately what you already input in the blanks below the section. Please note presenter's name will be shown with underline.
- The body of the abstract should be written within
3,500 bytes( A warning message window will pop-up when
exceeded). The total bytes of the present abstract can be seen
in the 'present condition of text input' item. It will count only
letters.
- The body of each abstract must contain the 4
designated headings: "Background:", "Methods:", "Results:", and "Conclusion:". Add a line space between paragraphs. No substitute headings, no combining headings, no abstracts without headings.
- Authors do not need to mention 4 designated heading letters before inputting contents in the content input paragraphs. It will be shown on the abstract automatically.
- Do not begin sentences with numerals.
- Spell out all abbreviations at first mention with abbreviation following in parentheses.
- For p value, "p" should be lowercase, roman. Do NOT capitalize of italicize.
- Authors should provide city and state (in parentheses) for manufactures cited within their abstract. Authors should supply generic drug name (in parentheses) after the brand or trade name. Do not use ® or ™ for brand names.
- Never use the underlining feature to denote the following symbols: ±, ≤, ≥.
- Space should appear around symbols and between number value and unit of measure (e.g., 3 ± 4, not 3±4; 3 mg, not 3mg).
- All measurements must include unit of measure (e.g., systolic blood pressure "140
mm Hg", not just "140"). Use Systeme Internationale (SI units).
- Express ejection fractions as a number (decimal) not as a percentage (e.g., 0.40, not 40%).
- Authors should use the word "table" or "figure" in text to refer to any table or graphic that may be included in their abstracts.
- Do not include references, credits or grant support.
- The BODY can be copied & pasted from other *.doc files, but you should confirm whether the special characters are not broken after the submission. If the characters are broken, you should input them directly. For special characters, click the [T*] button on the upper menu in the 4 designated heading.
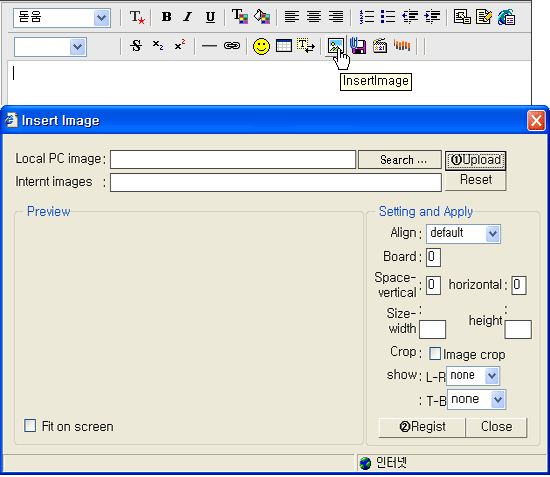
- Please click the appropriate buttons(
 , ,  ) on the upper menu and then insert where you want to insert Table or Figure. ) on the upper menu and then insert where you want to insert Table or Figure.
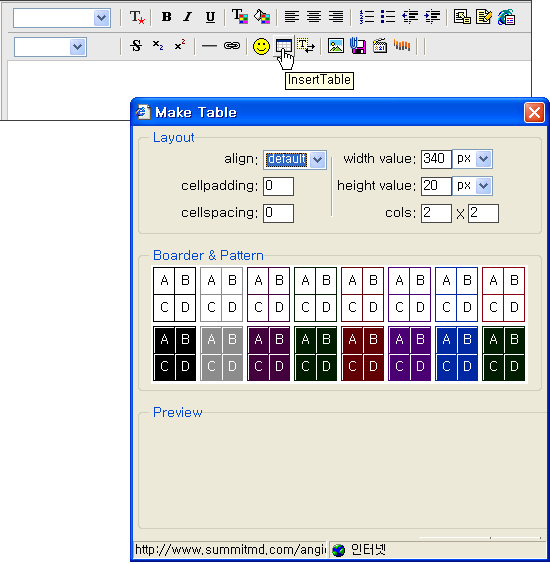
- Tables must have no more than 3 horizontal rules. No vertical rules of grid style tables. Superscript footnote symbols to table should be used in the following order: *, †, ‡, §,
ll, ¶, #. Do NOT use letters.Tables must have no more than 3 horizontal rules.
- You can copy & paste TABLE (only MS-WORD files are available), but you should confirm whether the special characters are not broken. You can click and select the special character button (T*) when you need the special character.
- The format of Figures should be submitted by jpg
or gif.
- You can adjust registered Figure.
- Note that we must reduce figures to a width of no more than 19 picas in the journal.
* All the information on the abstract is being sent to your e-mail address. If adjustment needed, login the page and adjust.
|






